As Indicated by Lewis Structures Which of the Following Species
Draw a skeletal structure for the following molecule. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom.
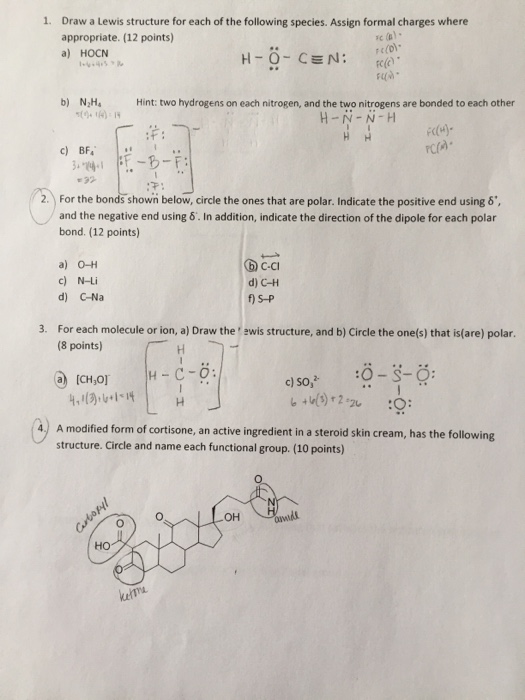
Solved 1 Draw A Lewis Structure For Each Of The Following Chegg Com
If the bulb contains 0001 mole of Ozg then find the volume of liquid water.
. B. As indicated by Lewis structures which of the following would probably not exist as a stable molecule. 6 After drawing the Lewis dot structure of HCOOH pick the INCORRECT statement from the following.
When appropriate draw all applicable resonance structures. B N 2 H 6. B Lewis structures with large formal charges eg 23 andor -2-3 are preferred.
How many electrons are shared between carbons 1 and 2. One point is earned for each correct structure. With reference to species A drawn below label compound B as an isomer a.
A In a Lewis structure the number of valence electrons shown is one more for each negative charge. The first atom in the formula is the central atom so the C atom in COS would be the central atom. The molecule ClO2 cannot be accurately described by a Lewis Structure.
Lewis Structure of CO2. The number of valence electrons for an atom is the number of electrons in the outer energy level shell of the atom. As indicated by the Lewis structures which of the following species could probably not exist as a stable molecule.
For species in which formal charges are not all zero determine the nonzero formal charges on the relevant atoms. CH 3 2 CCHCH 2 4 CH 3 15. The best Lewis structure for sulfuric acid has zero formal charges sulfur as the central atom.
D The O-H bond is a single bond. Chlorines electron configuration is 2-8-7. Oxalic acid H 2 C 2 O 4 is a poisonous compound found in rhubarb leaves.
A NH 3. The Lewis electron dot structures of a few molecules are illustrated in this subsection. B The carbon has a lone pair.
Since it is bonded to only one carbon atom it must form a double bond. Ssure of water is 28 mm of Hg. Do not add any more atoms.
Drawing Lewis Dot Structures for Atoms and Ions A. B On the basis of the Lewis structures drawn in part a answer the following questions about the. Identify the correct lewis symbol from the following.
The hybridization of the central atom S in SF_4 is Which of the following compounds has the smallest lattice energy. The diatomic molecule Cl2 is an example of a polar molecule. Using the following data reactions.
Given that R 0082 L atm mol K. Under Exact indicate whether the angle you selected is exact or only approximate. B The central atom is typically the atom with the.
Oxygen contains 6 valence electrons which form 2 lone pairs. CH 3 CH 2 2 CO 2 CCH 3 3 14. Correct option is.
As indicated by Lewis structures which of the following species could probably not exist as a stable molecule a. View the full answer. 32 Choose the INCORRECT statement.
E The C-H bond is a single bond. C Both oxygens have two lone pairs. A PH3 B NH4 C O3 D SO3- E HCN.
SrF2 As indicated by Lewis structures which of the following species could probably not exist as a stable molecule. The arrangement of atoms in several biologically important molecules is given here. AsindicatedbyLewisstructureswhichofthefollowingspeciescouldprobably from CHEM 180 at Orange Coast College.
31 As indicated by Lewis structures which of the following molecules would probably be unstable. At his temperature the vapour pre. A NHb N2H2 c NzHd N2H60 N204.
Write a Lewis structure for the phosphate ion PO 4. A The oxygen not also bonded to hydrogen has a double bond to carbon. Draw the Lewis Structures for each of the following species to answer the questions.
The bonds in LiF have a more covalent character than those in F2. NH_3 N_2H_2 N_2H_4 N_2H_6 N_2O_4 In CI_2CO the electron pairs are located about the central carbon atom in a arrangement pyramidal tetrahedral trigonal planar trigonal bipyramidal square planar The molecular shape. As indicated by Lewis structures which of the following species could probably not exist as a stable molecule.
Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Based on electronegativity trends in the periodic table predict which of the following compounds will have the greatest ionic character in its bonds. Which of the following species is best described by drawing resonance structures.
A NH3 B N2H2 C N2H4 D N2H6 E N2O4. As indicated by Lewis structures which of the following species could probably not exist as a stable molecule. The best Lewis structure for phosphoric acid has zero formal charges phosphorus as the central atom and no bonds between P and H.
C SF 4. As indicated by Lewis structures which of the following species could probably not exist as a stable molecule. As indicated by Lewis structures which of the following species could probably not exist as a stable molecule a.
The central atom of this molecule is carbon. A Draw the Lewis structure electron-dot diagram of each of the four species. The amino acid serine.
Lewis dot structure for an atom of chlorine is. There is a single bond between the two carbon atoms each hydrogen atom is bonded to an oxygen atom and each carbon is bonded to two oxygen atoms. Draw a valid Lewis dot structure and determine the VSEPR molecular geometry for each central atom for each of the following.
C The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms. Given the following Lewis structure. Neglect volume of U-tube manometer.
Show all valence electrons in your structures. As indicated by Lewis structures which of the following species could probably not exist as a stable. A A Lewis structure in which there are no formal charges is preferred.
D CH 2 F 2. Draw the Lewis structure for oxalic acid. Convert the following condensed formula to a Lewis structure.
A closed end manometer is filled with Hg as shown in the following diagram at 27C and volume of the bulb is 150 mL. As indicated by Lewis Structures which of the following species could probably not exist as a stable molecule.
Chem 101 Octet Rule Violations
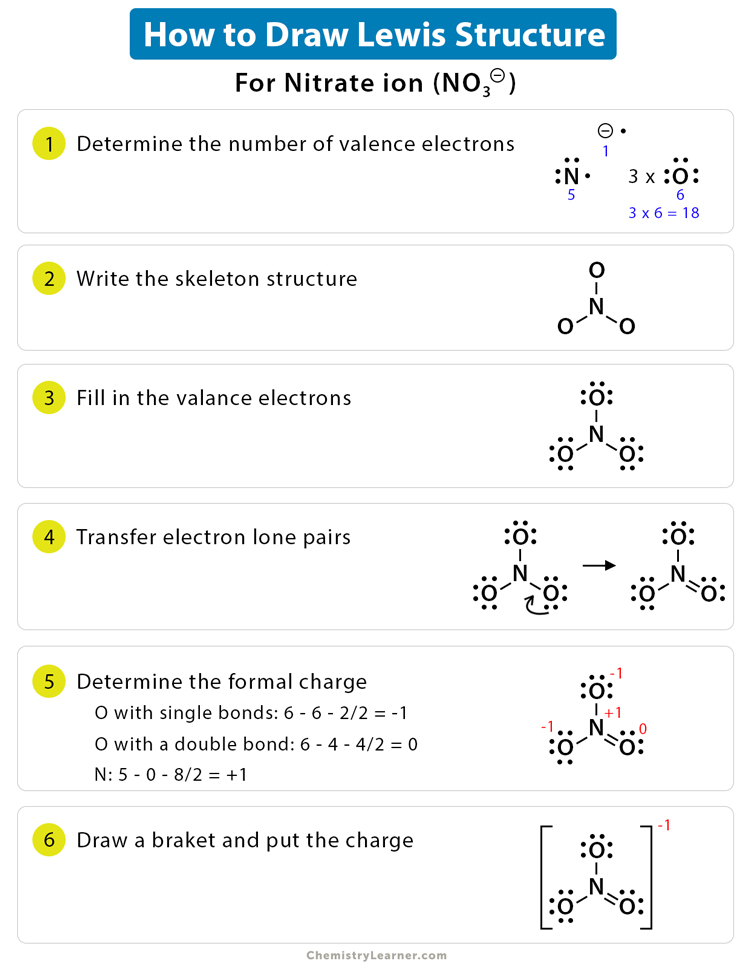
Lewis Dot Structure Definition Examples And Drawing
Lewis Structure Of N2o4 Youtube

Scn Lewis Structure How To Draw The Lewis Structure For Scn Thiocyanate Ion Youtube

Po4 3 Lewis Structure The Phosphate Ion Youtube
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Chemistry Help Molecular Shapes
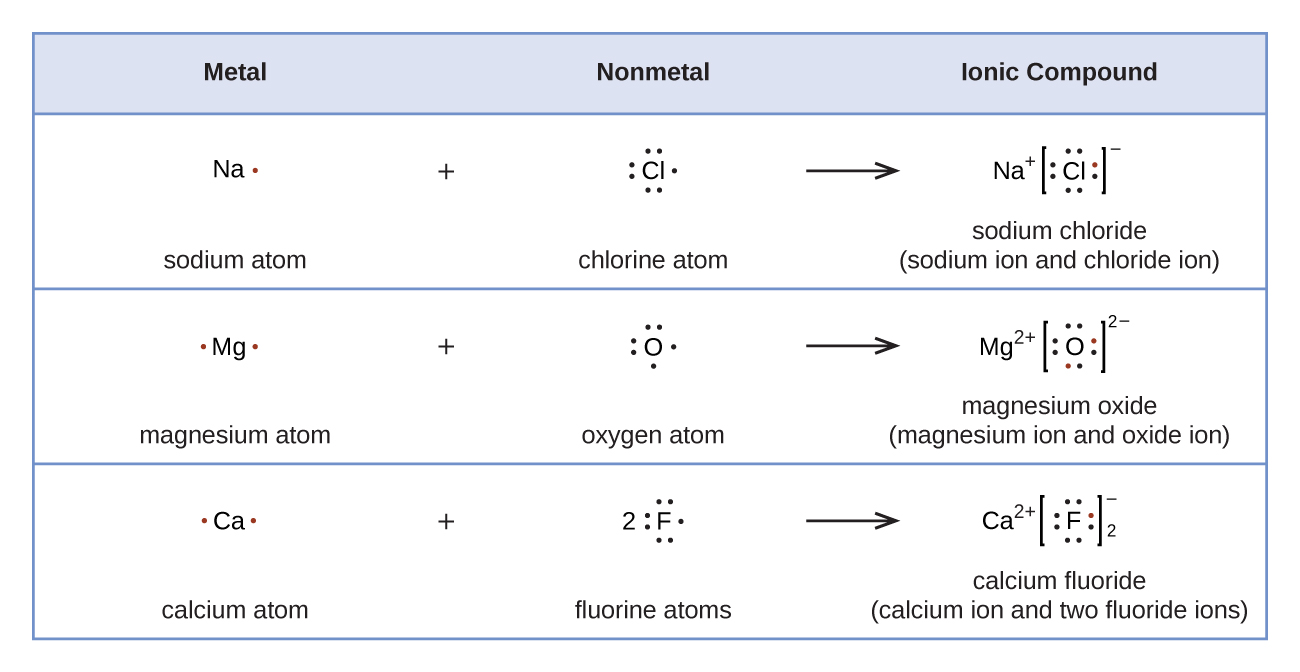
7 3 Lewis Symbols And Structures Chemistry
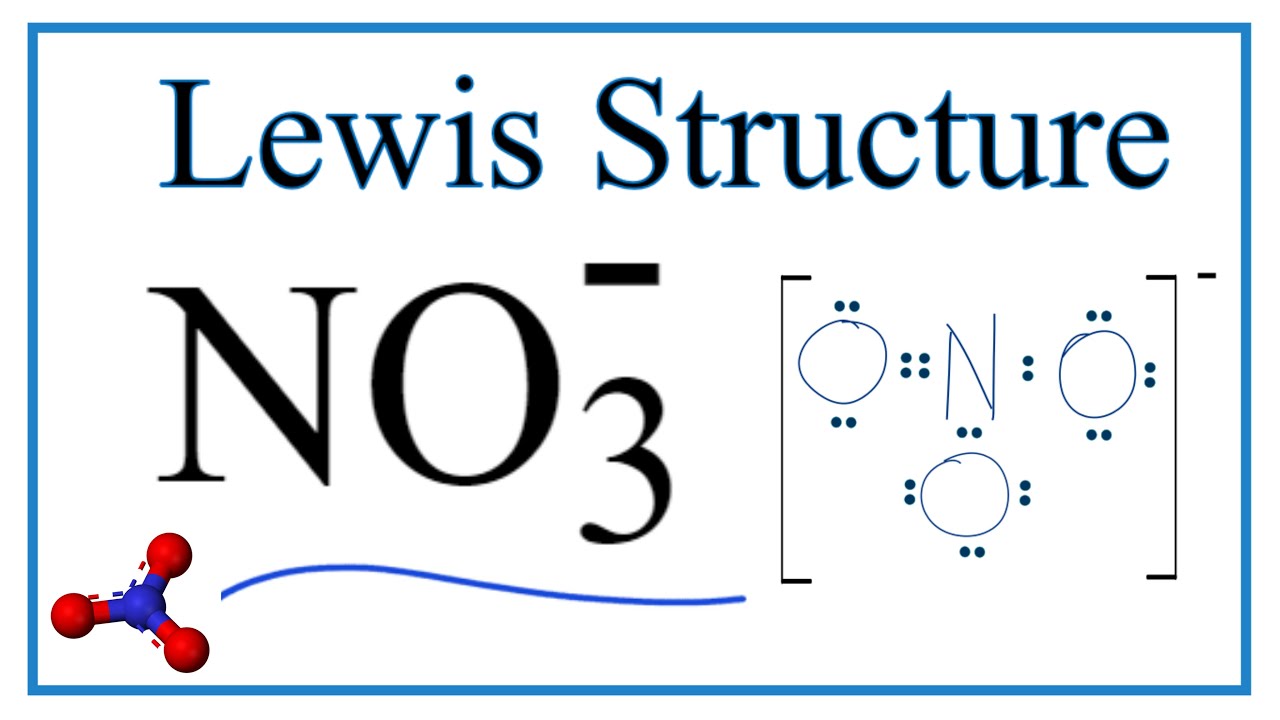
Lewis Structure For No3 Nitrate Ion

Lewis Structures For Covalent Molecules Step By Step Youtube
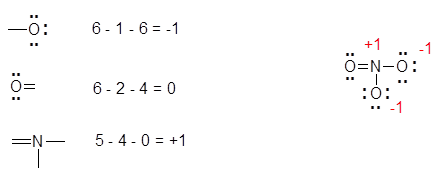
Lewis Structures Chemistry Libretexts
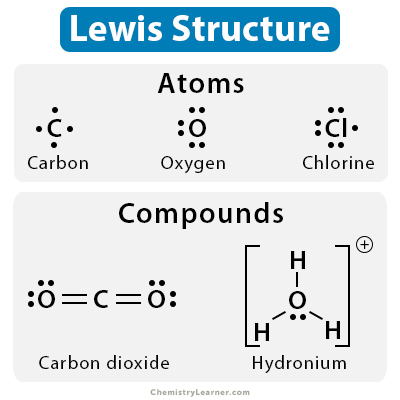
Lewis Dot Structure Definition Examples And Drawing
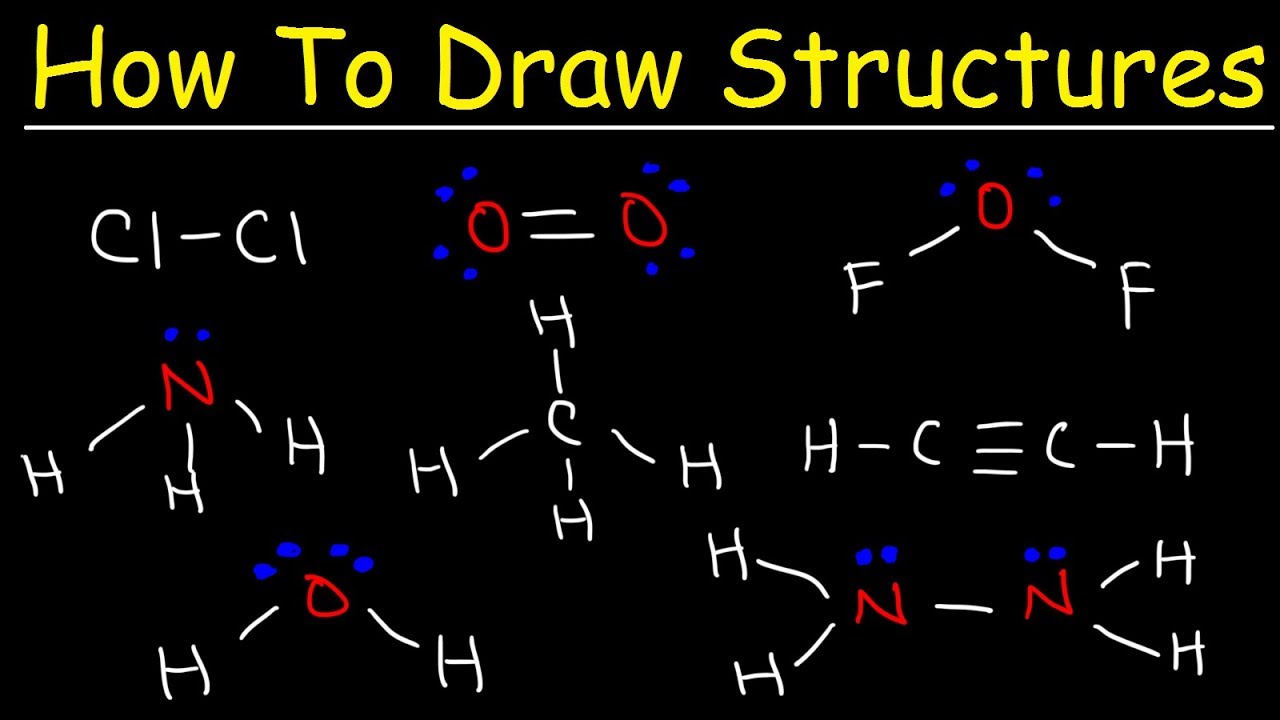
How To Draw Lewis Structures Youtube

Lewis Dot Diagrams Of The Elements Teaching Chemistry Chemistry Jokes High School Science
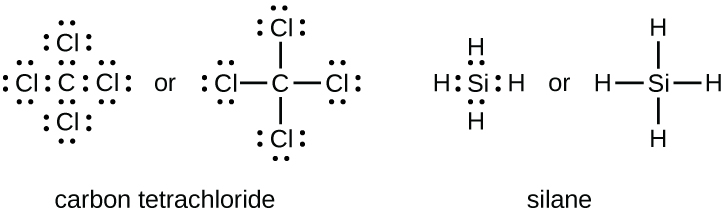
7 3 Lewis Symbols And Structures Chemistry
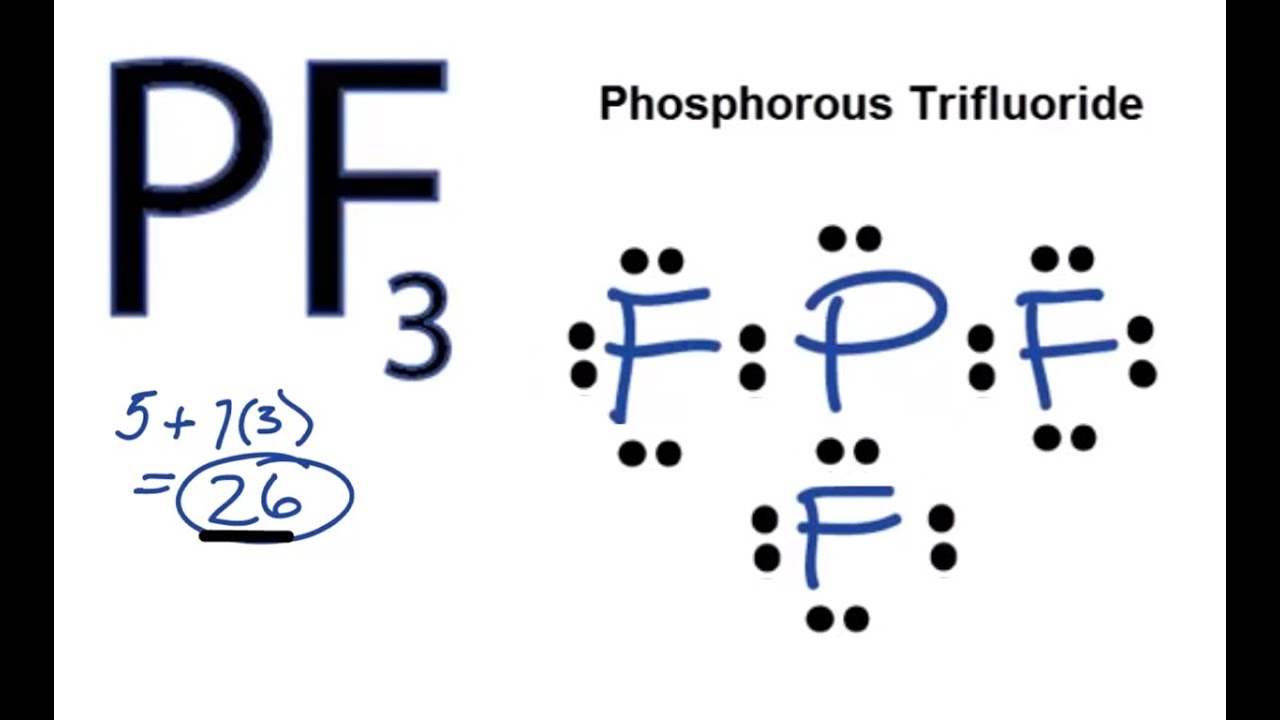
Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
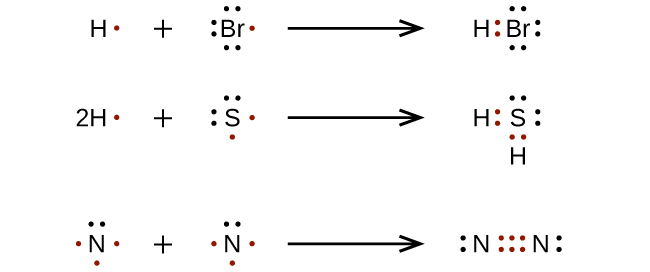
Comments
Post a Comment